The most common AEs reported in 5%–25% of patients across 5 clinical studies2†

Instillation-site
irritation
Dysgeusia

Reduced
visual acuity
“Facilitating realistic patient expectations is necessary to ensure patient satisfaction and compliance with their treatment.”3
—Shen Lee B, Kabat AG, Bacharach J, et al.
Xiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients2
*Based on a review of postmarketing safety data from July 2016 through July 2023.1
†In 5 clinical studies of dry eye disease with lifitegrast ophthalmic solution, 1401 patients received at least 1 dose of lifitegrast (1287 of whom received Xiidra). The majority of patients (84%) had up to 3 months of treatment exposure; 170 patients were exposed to Xiidra for approximately 12 months.2
MOST FREQUENT AEs (>5%) IN THE 12-MONTH SONATA SAFETY STUDY4‡§
The majority of treatment-emergent AEs (TEAEs) were mild to moderate in severity

No serious ocular AEs were reported4

Most AEs were mild to moderate in severity4

Discontinuation rates for Xiidra were low4||
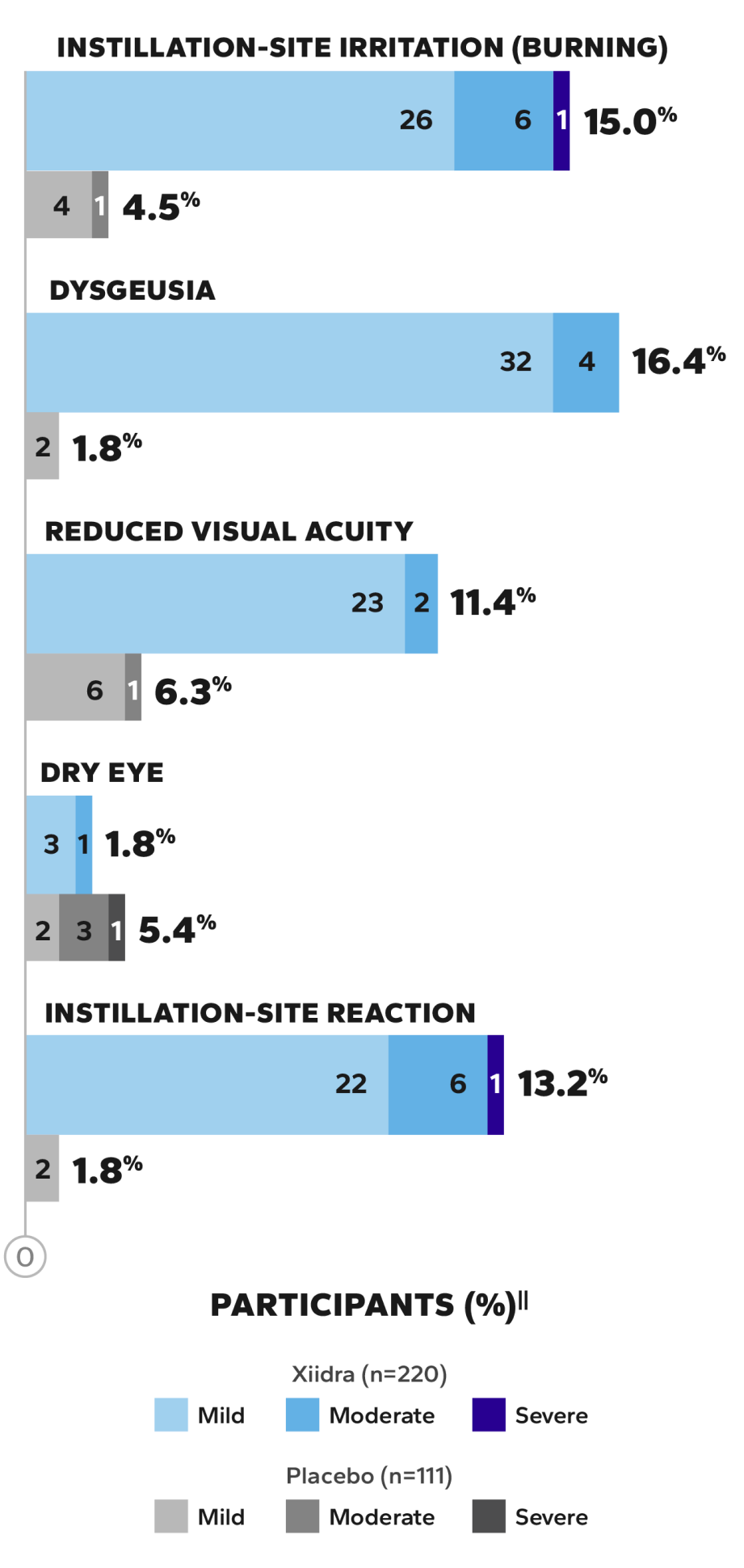

Values inside bars=number of participants.
‡The 1-year, multicenter, randomized, prospective, double-masked, placebo-controlled, phase 3 SONATA study evaluated the safety of Xiidra compared to vehicle in 331 adult patients who had a self-reported history of dry eye disease. Artificial tear use was assessed as an exploratory endpoint.4
§Adverse events occurring in >5% of participants in either treatment group.4
||In the SONATA study, a total of 24 participants (Xiidra, 8.2% [18/220] vs vehicle, 5.4% [6/111]) had at least 1 ocular treatment-emergent adverse event (TEAE) and 13 participants had at least 1 nonocular TEAE (Xiidra, 4.1% [9/220] vs vehicle, 3.6% [4/111]) that resulted in discontinuation.4
¶Percentage value indicates the proportion of participants who experienced each type of adverse event.4
Low Discontinuation Rates4,5#
Among participants treated with Xiidra in the SONATA safety study and a pooled analysis of four 12-week studies

discontinued due to instillation-site reaction and dysgeusia5

discontinued due to instillation-site irritation or pain5

discontinued due to reduced visual acuity4
Patients taking Xiidra had the lowest discontinuation rate after their first Rx compared with cyclosporine4¶
~8 out of 10 patients remained on Xiidra after 1 year in the SONATA safety study4**
In addition to potential AEs,
every patient prescribed Xiidra should know
THE COMFORT OF XIIDRA MAY IMPROVE OVER TIME5,6
Drop comfort score improvements were seen across 3 clinical trials from 2 weeks to 12 weeks at 3 minutes post instillation5,6††


The most exciting part about Xiidra is the patients that come back and want to still be on the medication.”
–Dr Preeya Gupta, Ophthalmologist, Triangle Eye Consultants
XIIDRA FORMULARY COVERAGE TOOL
Use this tool to help find coverage for Xiidra in your area
AE, adverse event.
#Overall, TEAEs that led to discontinuation were reported in 7.0% (90/1287) of participants receiving lifitegrast.5
**The 1-year, multicenter, randomized, prospective, double-masked, placebo-controlled, phase 3 SONATA study evaluated the safety of Xiidra compared to vehicle in 331 adult patients who had a self-reported history of dry eye disease. Artificial tear use was assessed as an exploratory endpoint.4
††Drop comfort was measured based on patient-reported scores from 0 (very comfortable) to 10 (very uncomfortable).5
‡‡Based on MMIT data from January 2026.
§§Xiidra significantly reduced symptoms of eye dryness at 2 weeks (based on Eye Dryness Score compared to vehicle) in 2 of 4 studies, with improvements observed at 6 and 12 weeks in all 4 studies.2
References
- Quint JM, Ratay M, Vittitow J, Brimer C. Safety of lifitegrast in patients with dry eye disease: analysis of a postmarketing database. Poster presented at: American Academy of Optometry (AAO) Annual Meeting; November 6-9, 2024; Indianapolis, IN.
- Xiidra. Prescribing information. Bausch & Lomb Inc.
- Shen Lee B, Kabat AG, Bacharach J, Karpecki P, Luchs J. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol. 2020;14:119-126. doi:10.2147/OPTH.S228838
- Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of lifitegrast ophthalmic solution 5.0% in patients with dry eye disease: a 1-year, multicenter, randomized, placebo-controlled study. Cornea. 2016;35(6):741-748. doi:10.1097/ICO.0000000000000803
- Nichols KK, Donnenfeld ED, Karpecki PM, et al. Safety and tolerability of lifitegrast ophthalmic solution 5.0%: pooled analysis of five randomized controlled trials in dry eye disease. Eur J Ophthalmol. 2019;29(4):394-401.
- Data on file. Bausch & Lomb Inc.
Indication
Xiidra® (lifitegrast ophthalmic solution) 5% is indicated for the treatment of signs and symptoms of dry eye disease (DED).
Important Safety Information
- Xiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients.
- In clinical trials, the most common adverse reactions reported in 5-25% of patients were instillation site irritation, dysgeusia and reduced




