In four 12-week, randomized, multicenter, double-masked, vehicle-controlled studies,
XIIDRA IMPROVED DRY EYE SIGNS AND SYMPTOMS1
Early relief that builds over time.
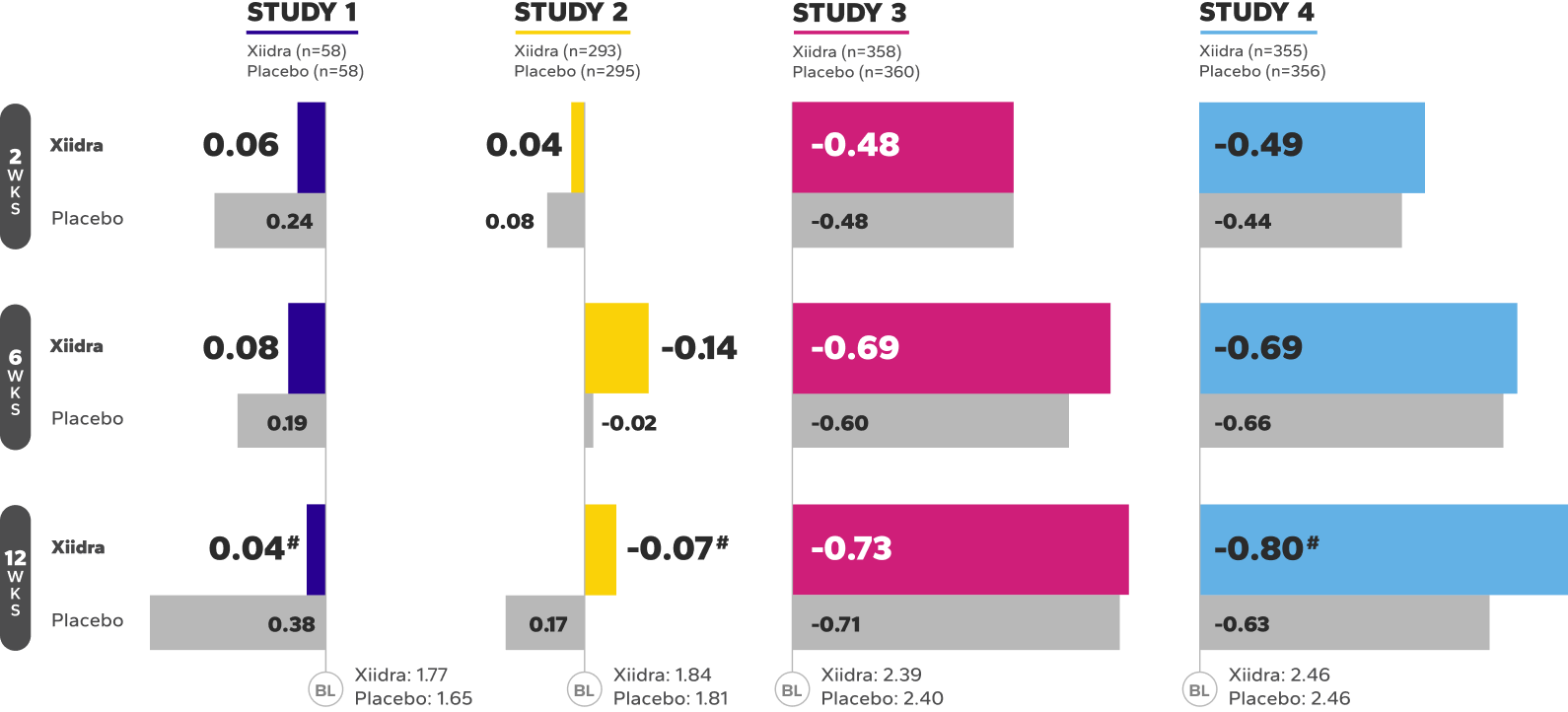
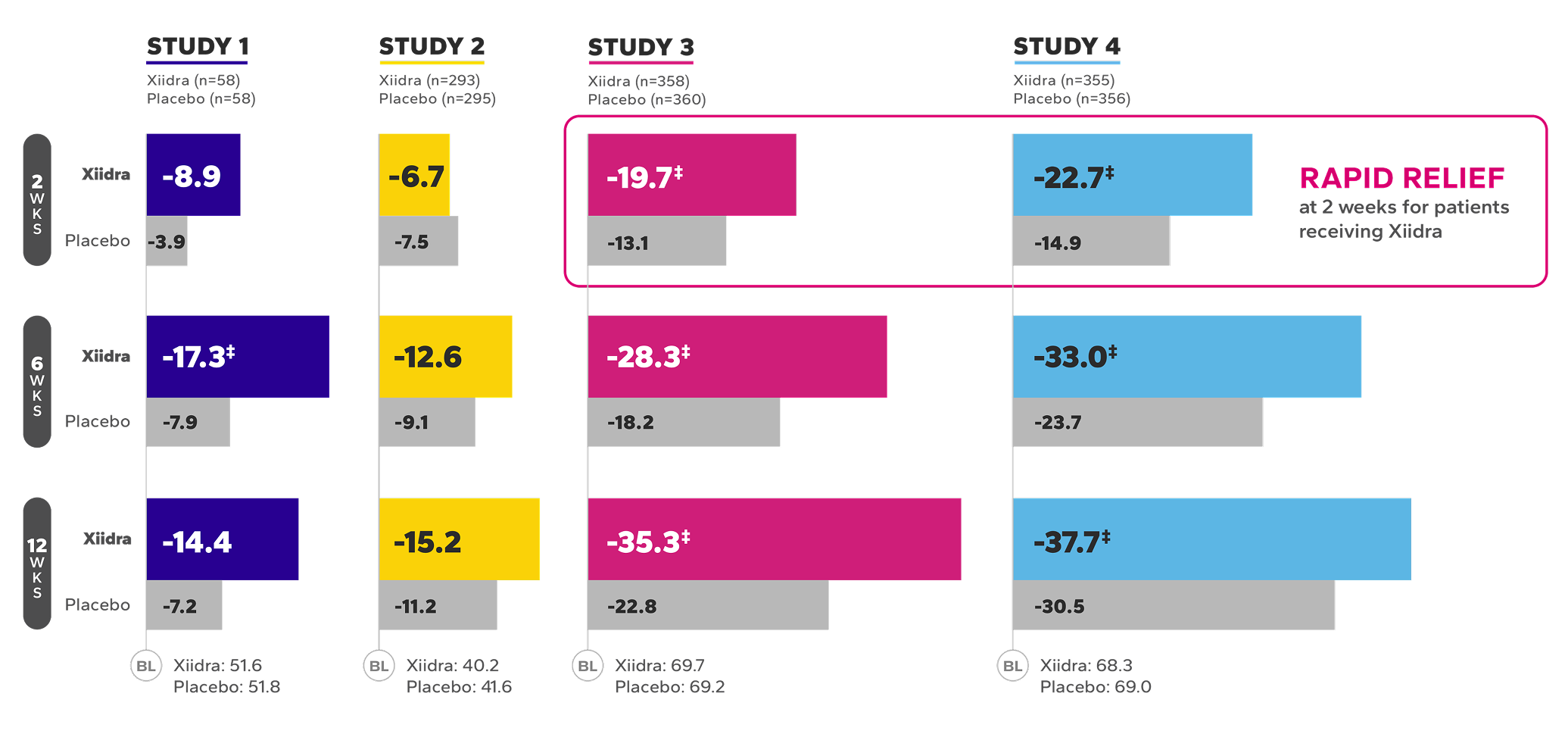
Xiidra significantly reduced symptoms of eye dryness at 2 weeks in 2 of 4 studies, with improvements observed at 6 and 12 weeks in all 4 studies1
Mean Change From Baseline in EDS in Four 12-Week Studies1*†
BL, baseline; EDS, eye dryness score; VAS, visual analog scale; WKS, weeks.
*In Study 1, Week 6 was a secondary endpoint; Study 3, Week 12 was a co-primary endpoint and Weeks 2 and 6 were ad-hoc endpoints; Study 4, Week 12 was the primary endpoint and Weeks 2 and 6 were secondary endpoints.
†EDS was rated by patients using a VAS (0=no discomfort, 100=maximal discomfort) at each study visit.
‡Indicates statistically significant improvement.
§Data from a pooled post-hoc analysis of Studies 3 and 4.2
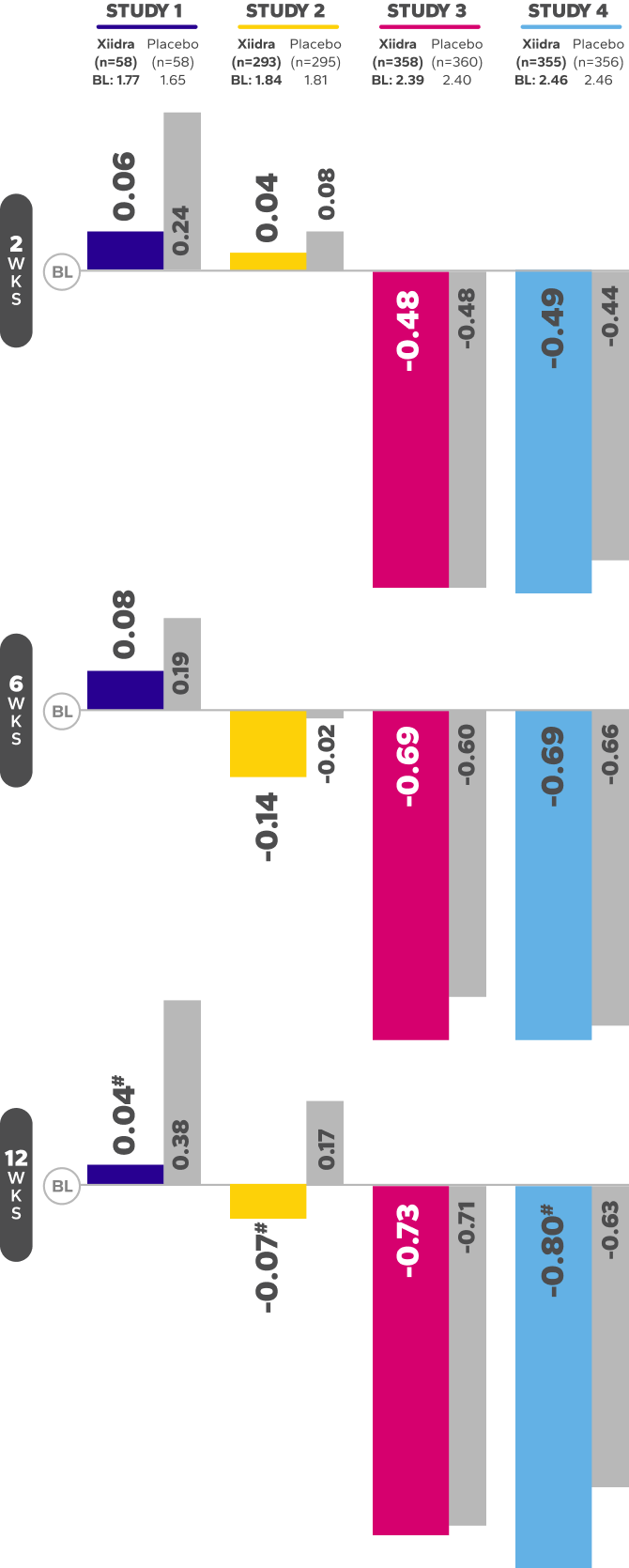
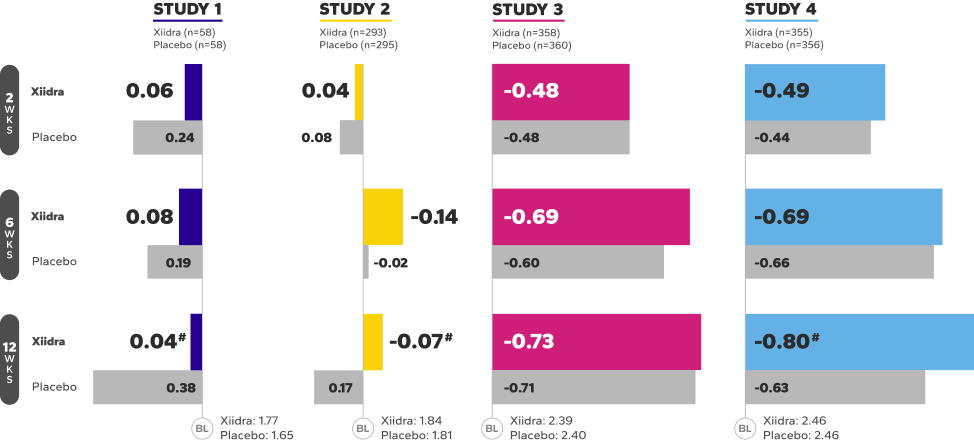
Xiidra Helped Improve the Ocular Surface by Reducing Corneal Staining
In 3 of 4 studies, a larger reduction in ICSS favoring Xiidra was observed at 12 weeks1
Mean Change From Baseline in ICSS in Four 12-Week Studies1||¶
BL, baseline; ICSS, Inferior fluorescein Corneal Staining Score; WKS, weeks.
||In Study 1, Week 12 was a secondary endpoint; Study 2 and 3, Week 12 was a co-primary endpoint; Study 4, Week 12 was an ad-hoc endpoint.
¶ICSS (0 = no staining, 1 = few/rare punctate lesions, 2 = discrete and countable lesions, 3 = lesions too numerous to count but not coalescent,4 = coalescent) was recorded at each study visit.
#Indicates statistically significant improvement.
The safety and efficacy of Xiidra were assessed in four 12-week, randomized, multicenter, double-masked, vehicle-controlled studies (N=2133). Patients were dosed twice daily (approximately 12 hours apart). Use of artificial tears was not allowed during the studies. The study end points included assessment of signs (based on ICSS on a scale of 0–4) and symptoms (based on patient-reported EDS on a visual analogue scale of 0–100).1
Based on analysis of covariance (ANCOVA) model adjusted for baseline value in Study 1, and ANCOVA model adjusted for baseline value and randomization stratification factors in Studies 2–4. All randomized and treated patients were included in the analysis and missing data were imputed using last-available data. In Study 1, one Xiidra-treated patient who did not have a baseline value was excluded from analysis. In Study 2, one vehicle-treated patient who did not have a study eye designated was excluded from analysis.1
In a single-center, prospective, open-label study of 40 patients,
Xiidra was observed to deliver relief for contact lens (CL) wearers5,6
Data should be interpreted with the study design and limitations in mind. No formal conclusions should be drawn.
The leading causes of CL
discontinuation are discomfort
and dryness7

CL WEARERS MAY
DISCONTINUE USE
OF THEIR LENSES8
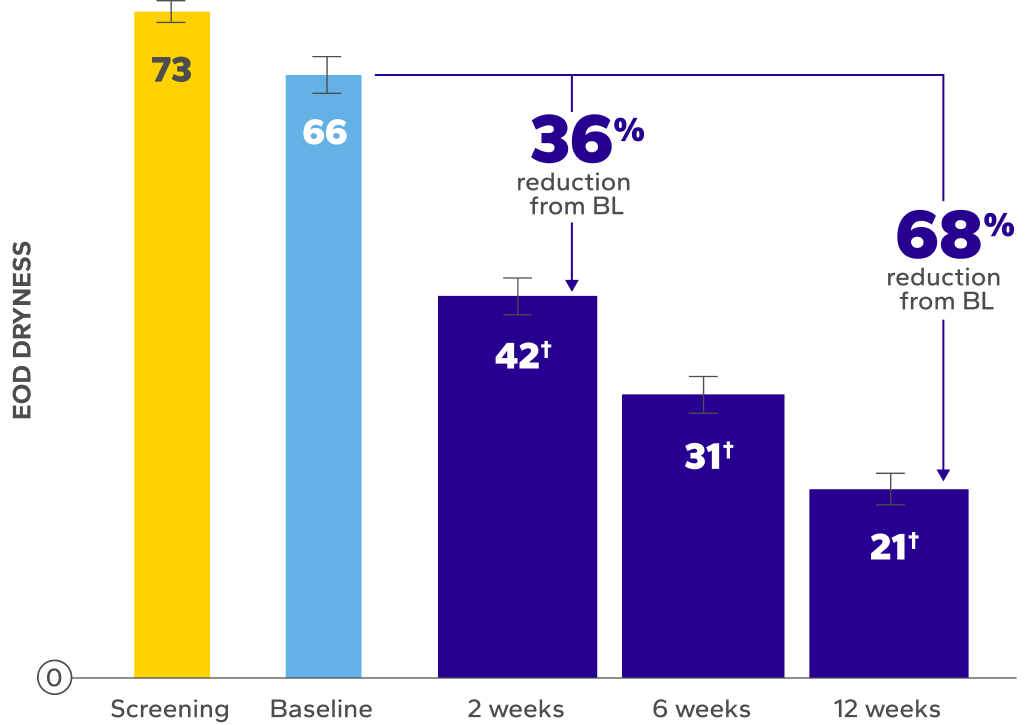
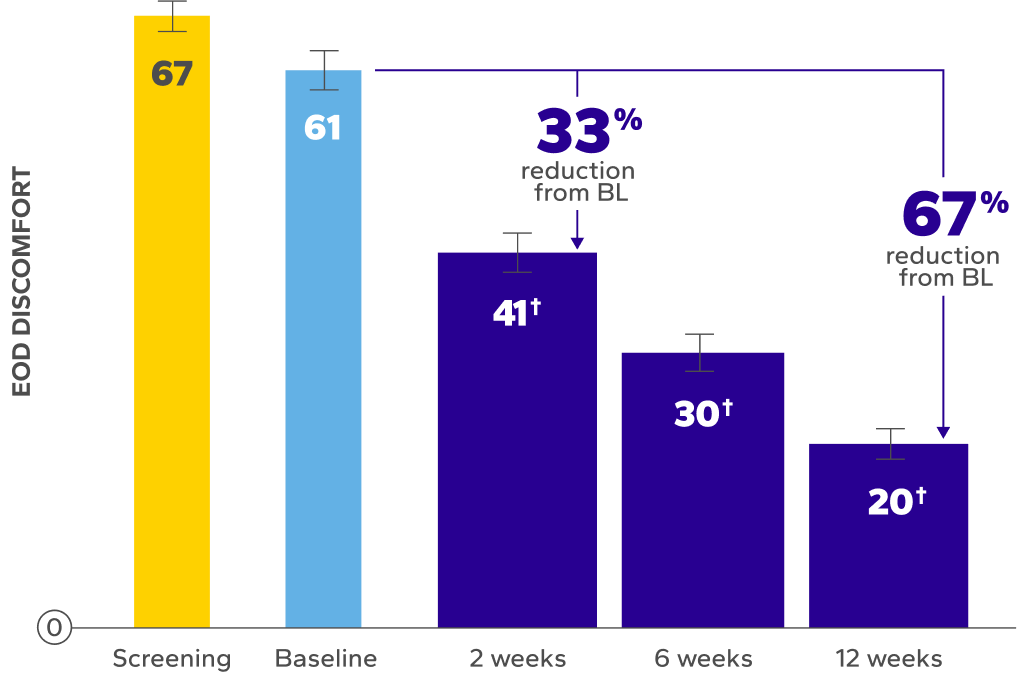
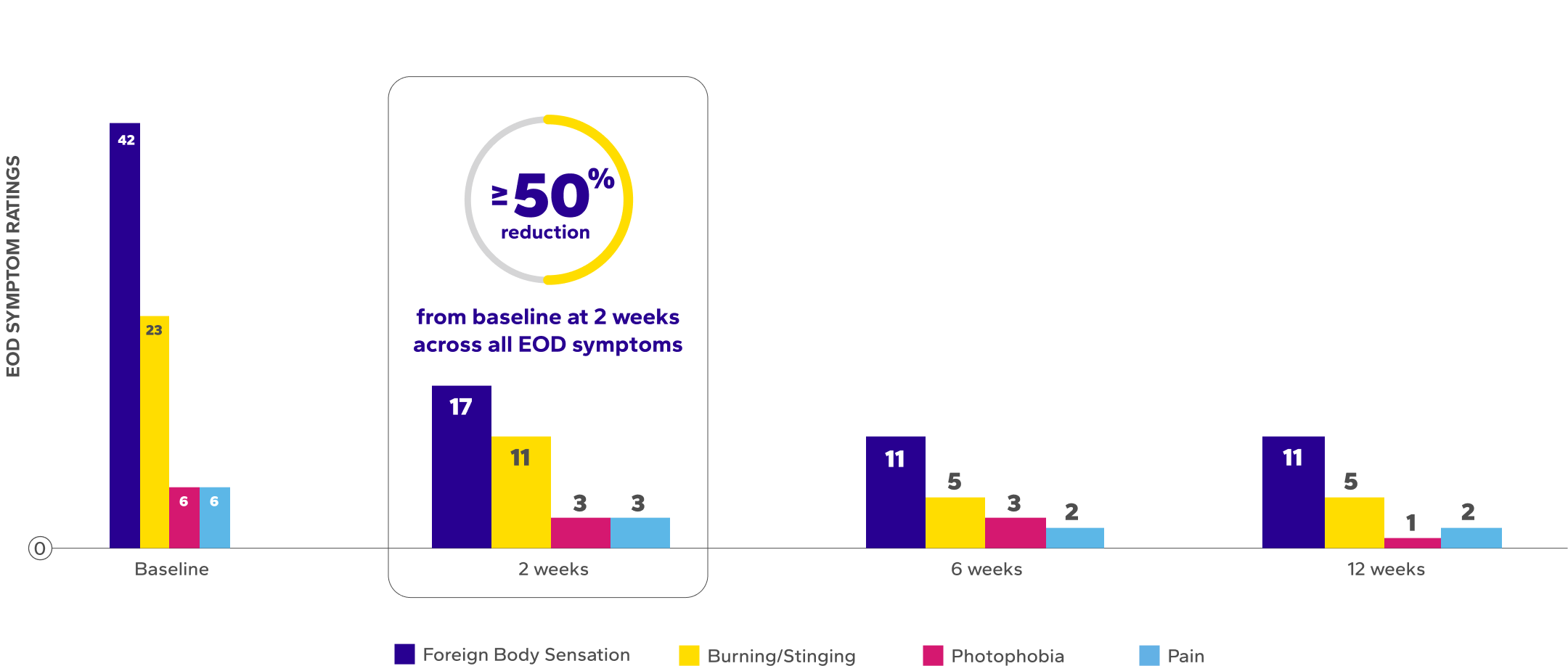
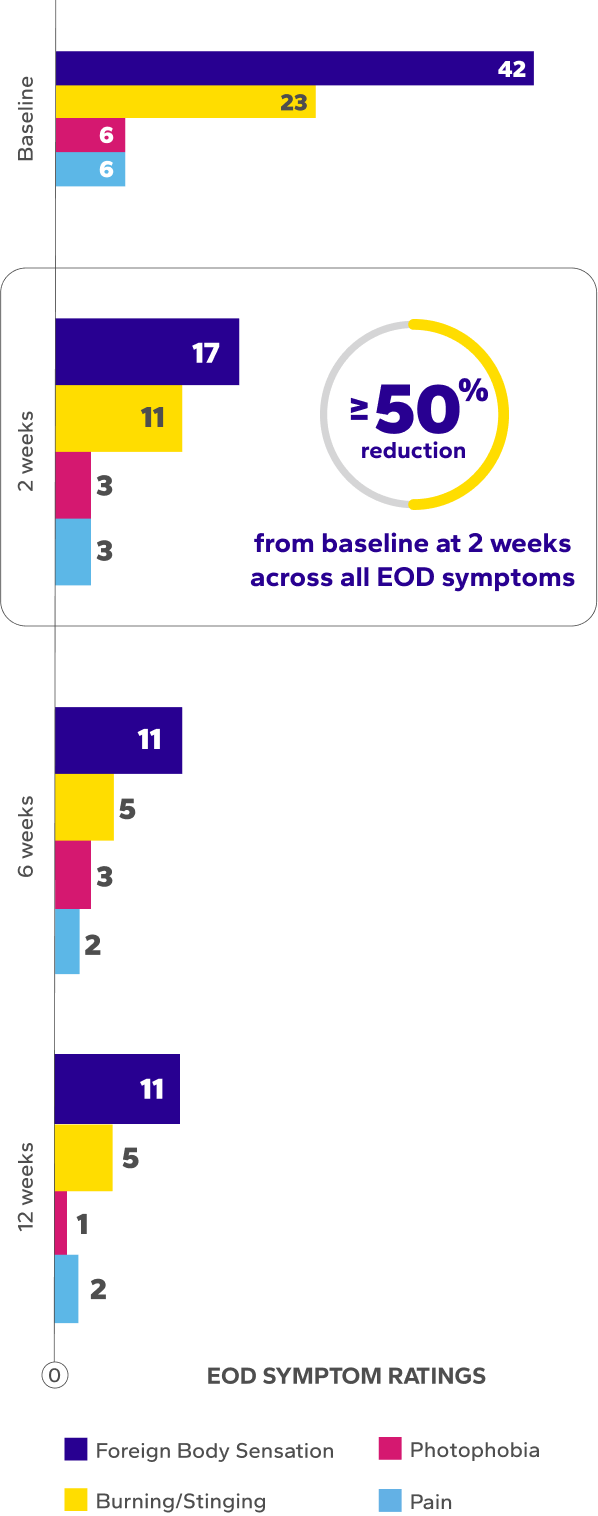
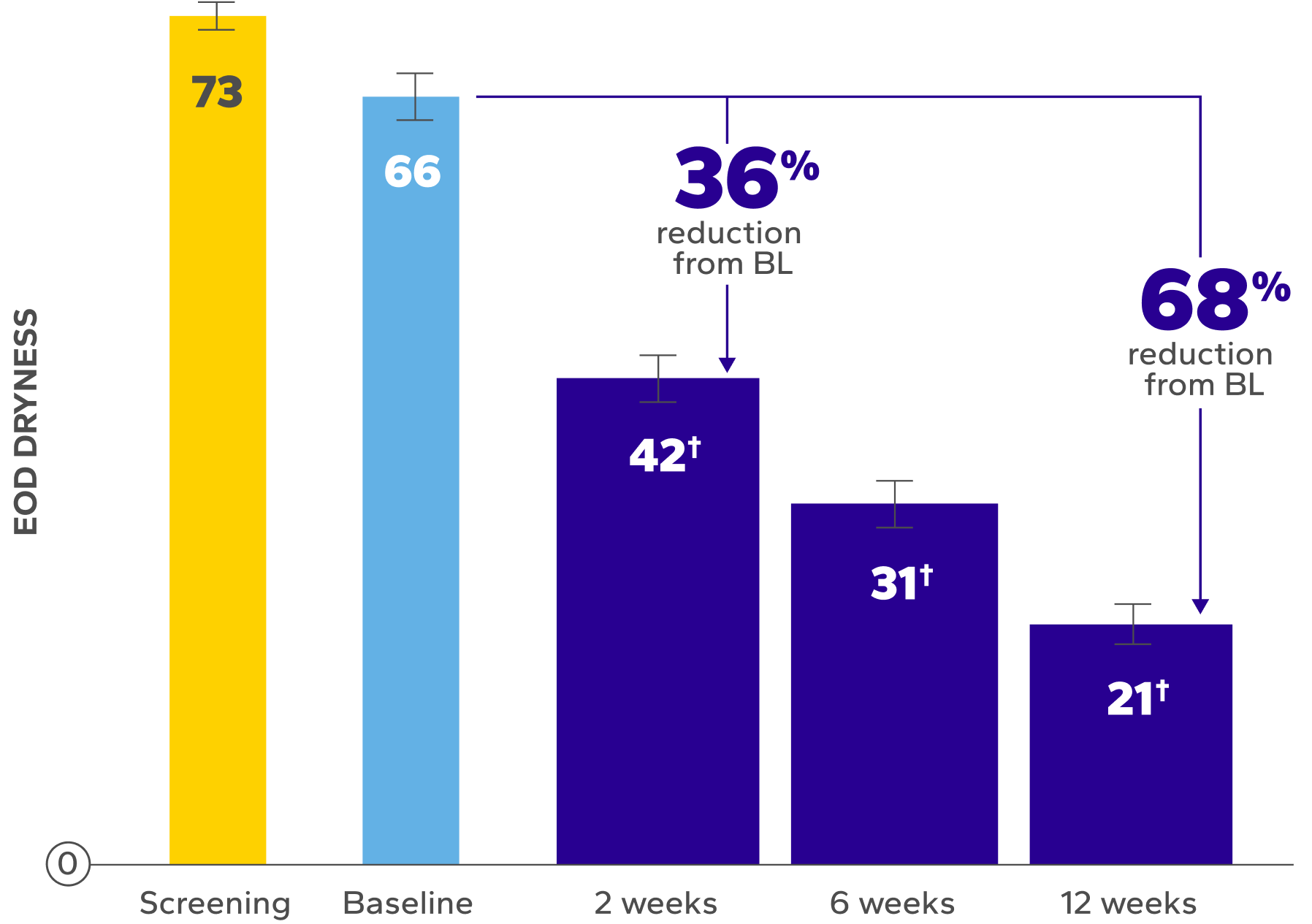
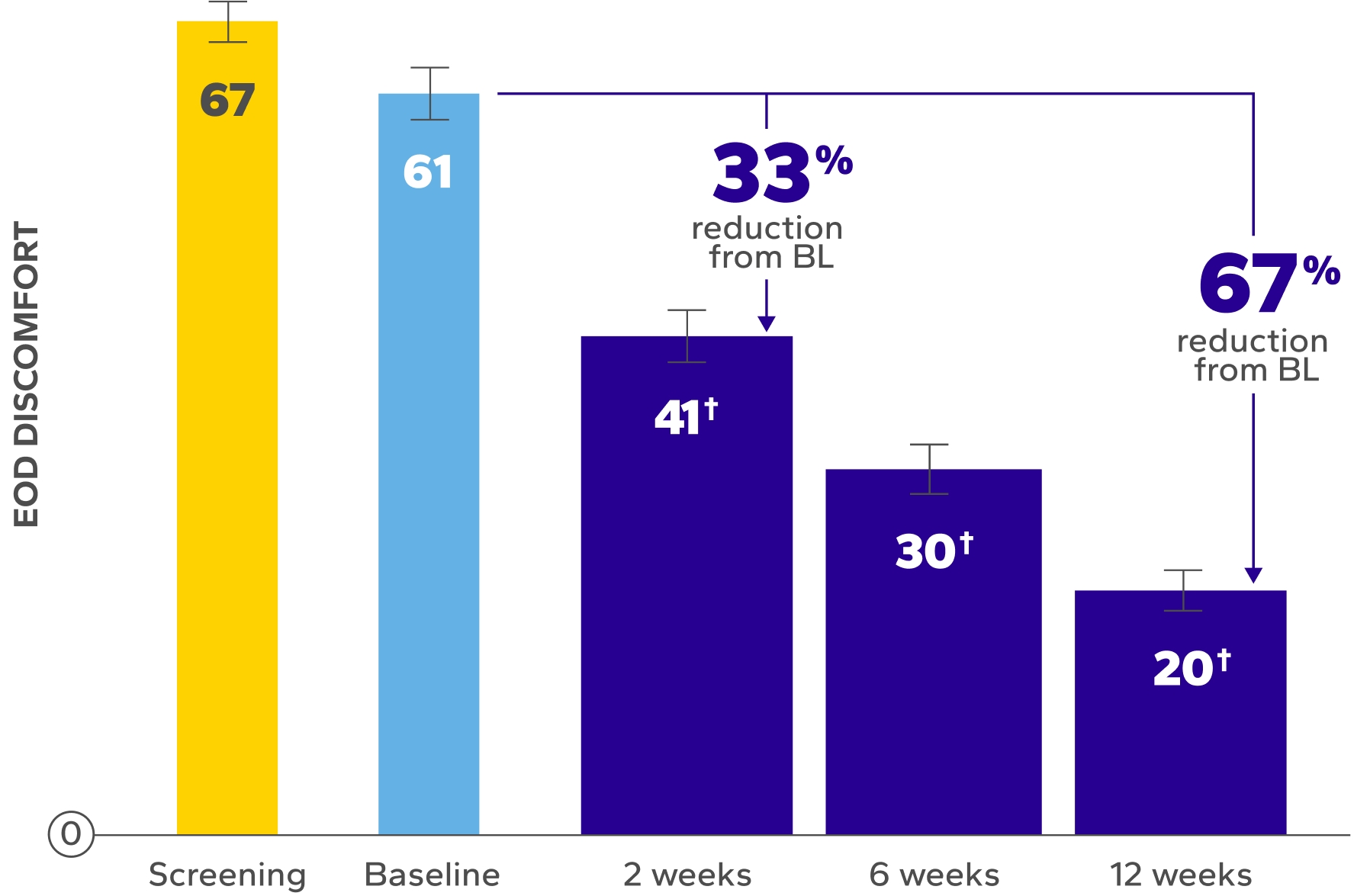
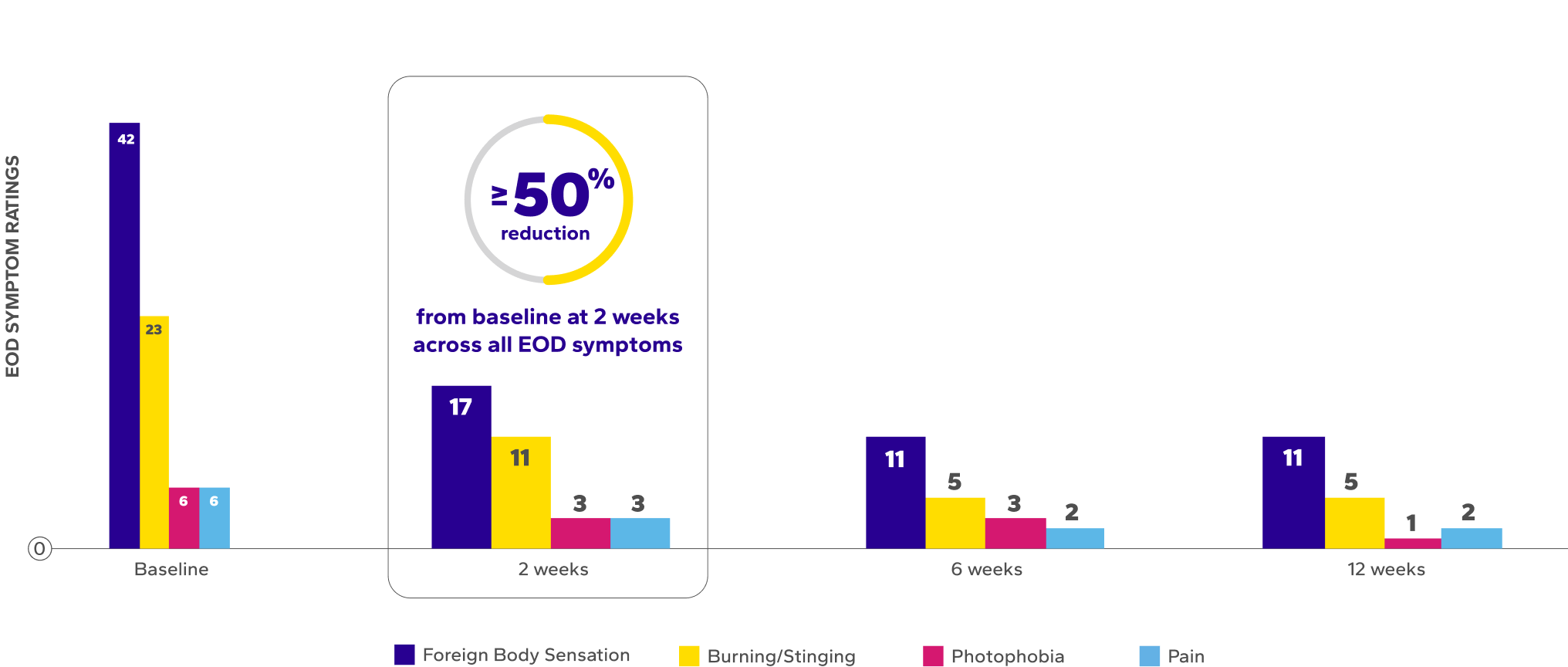
Significant Improvements in End-of-Day (EOD) Dryness and EOD Discomfort
Patients using Xiidra experienced significant reductions in EOD dryness and EOD discomfort vs baseline at all time points (P<0.01)5*
Habitual lenses worn by participants comprised 17 different lens types, including both reusable (35%) and daily disposable (65%) CLs.5
Xiidra was used per label, administered twice daily, and instilled at least 15 minutes before contact lens insertion.6
BL, baseline.
Errors are the standard error of the mean.
*EOD dryness and EOD discomfort were rated by patients using a VAS 0–100, with 100 being worst.
†Indicates significant difference vs baseline.
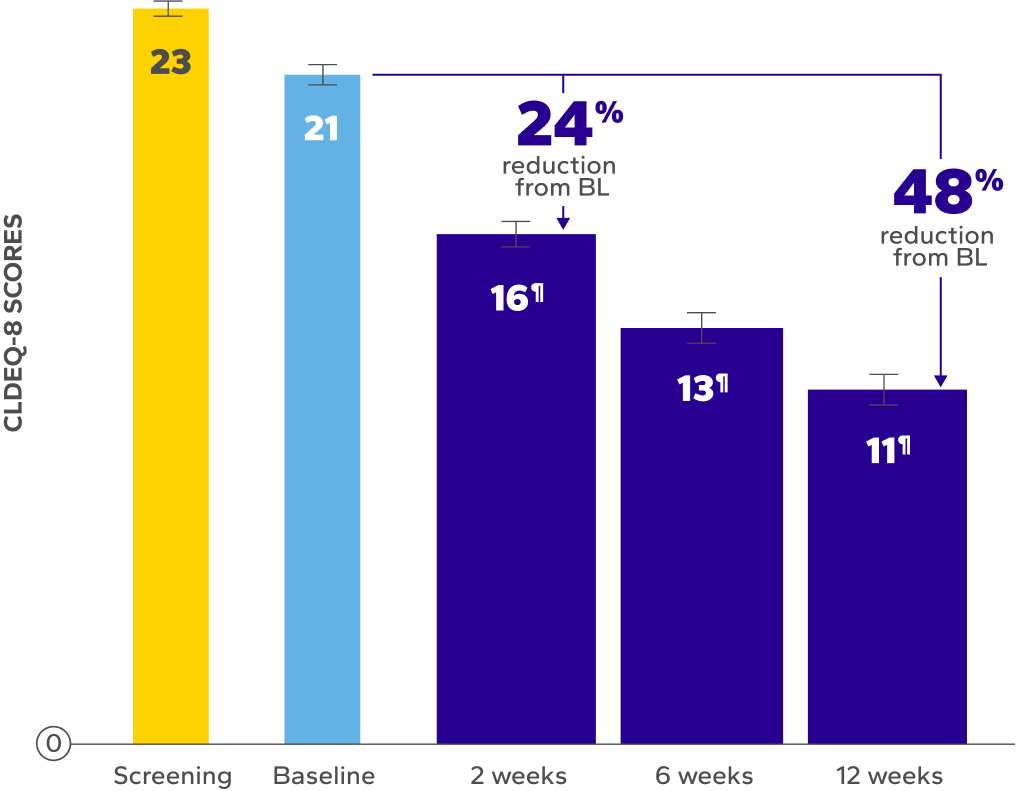
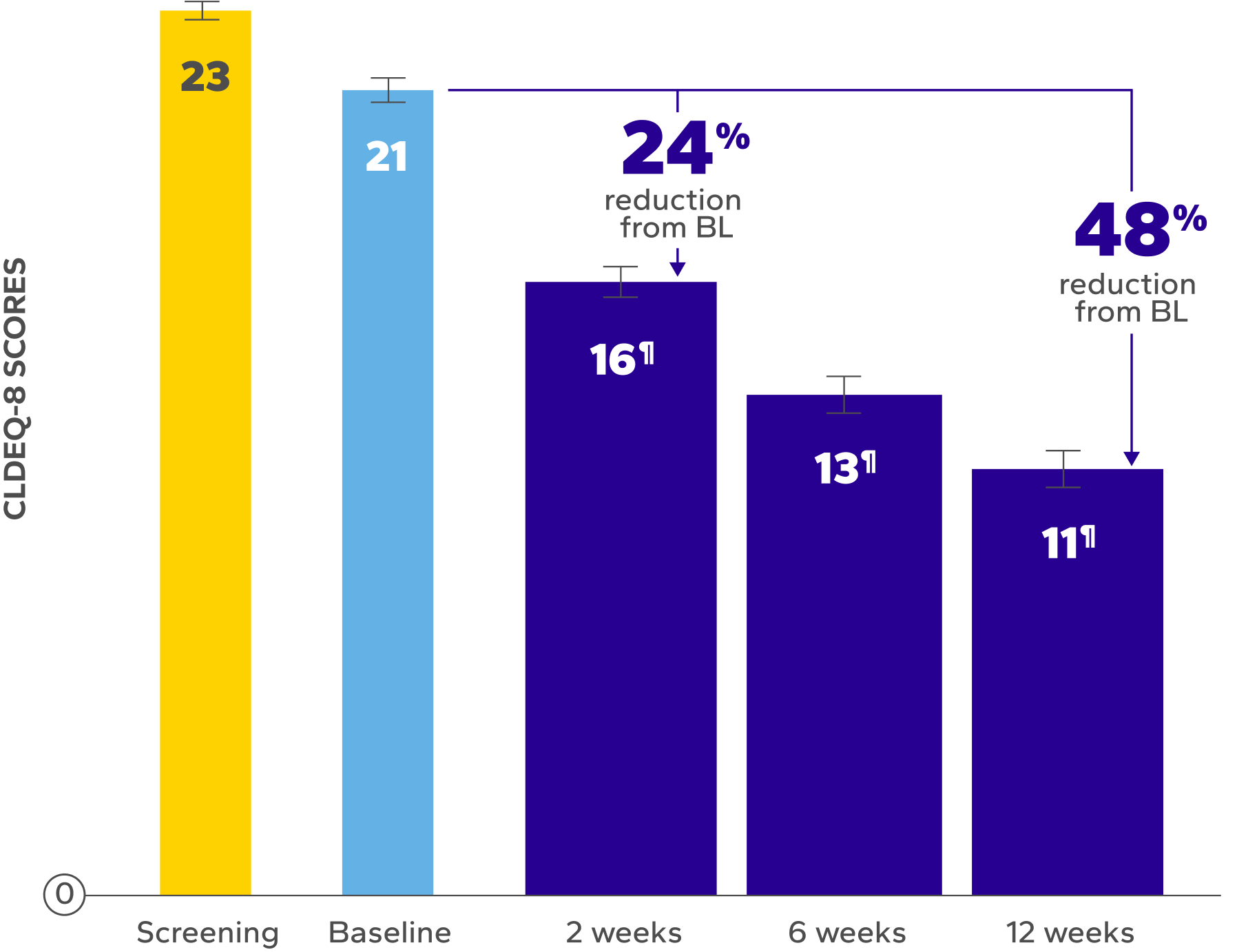
Significant Relief from Dry Eye Symptoms
Patients using Xiidra experienced significant reductions in dry eye symptoms vs baseline at all time points (P<0.01)5,6
Errors are the standard error of the mean.
of patients achieved a clinically meaningful improvement of at least 3 points in CLDEQ-8 scores vs their baseline score after 12 weeks6
Errors are the standard error of the mean.
BL, baseline; CLDEQ-8, contact lens dry eye questionnaire-8.
‡The CLDEQ-8 was administered at every visit. Participants circled the answer to each of the 8 questions that best corresponded to their experience with their habitual contact lenses for the past 2 weeks. Once completed, the answers were summed up to form an overall score (possible range 1–37).6
§Indicates significant difference vs baseline.
ǁDry eye symptoms were rated by patients using a VAS 0–100, with 100 being worst.
¶The range of VAS scores for EOD symptoms were as follows: burning/stinging at baseline (0–91), 2 weeks (0–73), 6 weeks (0–75), 12 weeks (0–62); foreign body sensation at baseline (0–94), 2 weeks (0–98), 6 weeks (0–82), 12 weeks (0–96); photophobia at baseline (0–77), 2 weeks (0–79), 6 weeks (0–77), 12 weeks (0–45); and ocular pain at baseline (0–85), 2 weeks (0–61), 6 weeks (0–49), 12 weeks (0–23).6
Safety Findings
Two treatment-related adverse events (AEs) were reported: significant tearing, leading to treatment discontinuation; and periorbital eyelid dryness. Both resolved upon discontinuing Xiidra. During the study, some participants reported transient dysgeusia and instillation-site irritation. Patients were counseled on common AEs of Xiidra, including dysgeusia; tearing; and intermittent irritation, discomfort, and blurred vision upon instillation. Prior knowledge of these AEs helped patients manage their experiences.6
Study Design
A single-center, prospective, open-label study of 40 patients who wear contact lenses and experience symptoms of eye dryness and contact lens discomfort evaluated whether symptomatic contact lens wearers achieve improvements in comfort and dryness when treated with Xiidra for 12 weeks. Patients attended a baseline and dispense visit and 3 follow-up visits after 2, 6, and 12 weeks. Study variables collected at all visits included EOD dryness and EOD discomfort ratings (0–100 VAS; 100=worst), CLDEQ-8 score and total and comfortable contact lens wear time. Xiidra was administered as 1 drop, twice daily (approximately 12 hours apart) into each eye, and contact lenses were removed prior to administration and reinserted 15 minutes following administration per the label. Participants included patients ≥18 years of age who were symptomatic habitual soft contact lens wearers who had a history of artificial tear (AT) or rewetting drop use at least once in the last 30 days and who were willing to use Xiidra twice daily (irrespective of CL wear) and to stop use of any habitual rewetting drops and/or ATs over the 12-week study.5,6
Study Limitations
A single-center, open-label study design with no control treatment arm. All participants had to have used ATs or rewetting drops at least once in the month prior to study start; only 30% of participants had used these eye drops twice per day (Xiidra dosing frequency), while the remaining 70% either had to increase (62%) or decrease (8%) their daily dosing frequency during the study. It is possible that the more frequent use of Xiidra as compared to previous drops could have contributed to the reduction in symptoms in some participants.6 Data should be interpreted with the study design and these limitations in mind. No formal conclusions should be drawn.
In a multicenter, prospective study of 100 eyes of 100 patients
Xiidra was observed to deliver relief before and after CATARACT SURGERY9
Data should be interpreted with the study design and limitations in mind. No formal conclusions should be drawn.

of patients undergoing screening for cataract surgery may have the hallmarks of dry eye9
Addressing dry eye before
surgery can help improve both
visual outcomes and patient
satisfaction after surgery10
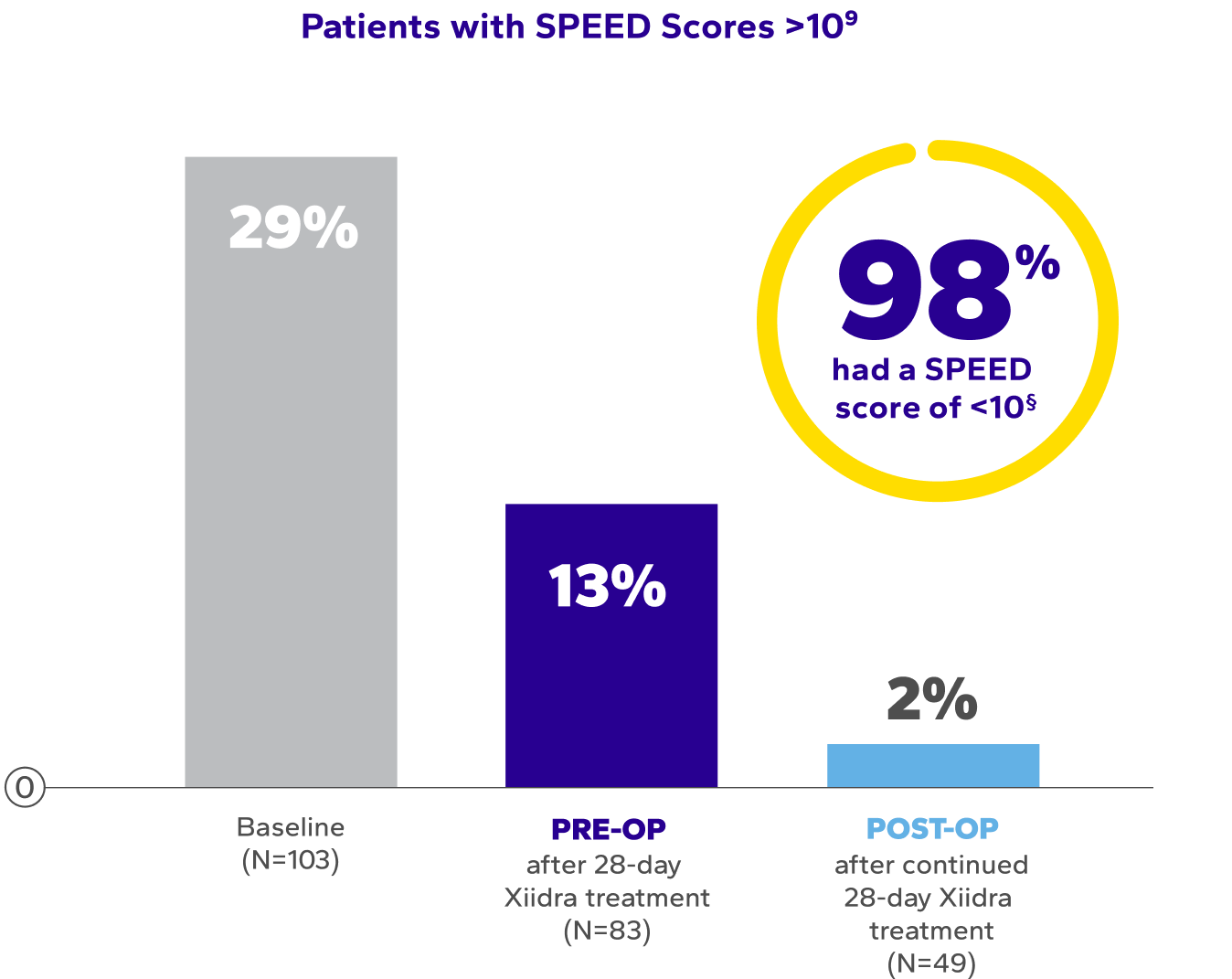
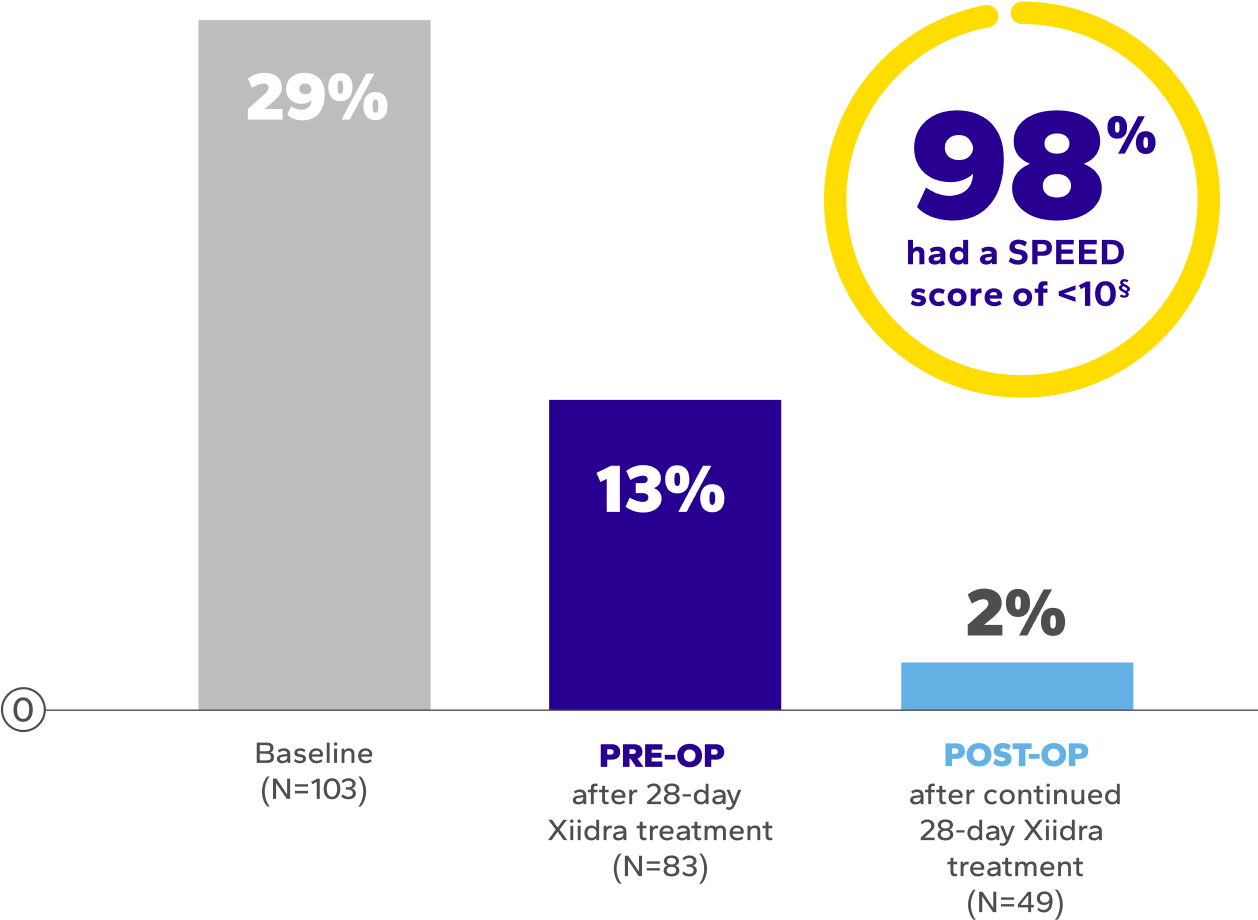
Significant Symptom Relief Pre- and Post-Surgery
Patients using Xiidra experienced significant improvements in symptoms after initial and continued treatment9*†‡
SPEED, Standardized Patient Evaluation of Eye Dryness.
*Data extracted from secondary outcomes. Results were consistent with pivotal trials.
†Dry eye symptoms were measured by SPEED scores. SPEED scores >10 are considered highly symptomatic of dry eye disease.9
‡The mean overall SPEED scores at baseline: 8.1 ± 6.6; after the initial 28 days of Xiidra: 6.3 ± 4.9; and after surgery continued Xiidra treatment: 4.0 ± 3.2 (P<0.0004, paired t-test).9
§Out of 103 patients enrolled in the study, only 49 patients completed postoperative Xiidra treatment.9
For nearly a decade—the ONLY
nonsteroid anti-inflammatory Rx that can start to deliver dry eye symptom relief in just 2 weeks1

Continued use of Xiidra led to
~2.5 ADDITIONAL HOURS OF COMFORTABLE wear time
While mean total wear time remained the same over time, patients taking Xiidra achieved significantly longer comfortable wear time vs baseline at all follow-up visits (baseline: 6.5 hours, 12 weeks: 9.1 hours; P<0.01)6
Significant and Sustained Improvement in Signs
Patients using Xiidra experienced improvement in dry eye signs after initial and continued treatment9||¶
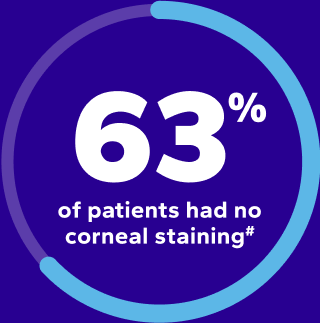

PRE-OP
after 28-day
Xiidra treatment
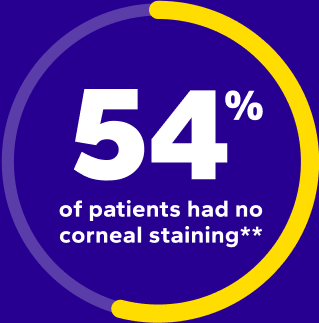

POST-OP
after continued
28-day Xiidra treatment
||About 30 days after surgery, 50 patients continued treatment with Xiidra for 28 days, and these patients had sustained improvements in ocular surface quality.9
¶At baseline, 60 (58%) patients had grade 1 stain, 40 (39%) had grade 2, and 3 (3%) had grade 3 or higher stain.9
#After 28-day treatment with Xiidra, 52 (63%) patients had no staining, 29 (35%) had grade 1, and 2 (2%) had grade 2 or greater staining.9
**After surgery and 28-day treatment with Xiidra, 27 (54%) patients had no staining, 21 (42%) had grade 1, and 2 (4%) had grade 2 (P<0.00001, paired t-test compared to baseline for both initial and continued Xiidra treatment).9
Statistically Significant Improvement in Biometry Accuracy
Patients using Xiidra experienced significant improvement in biometry accuracy after 28-day treatment9††
Accuracy of Predicted Final Refractive Outcome9






††Biometry performed before surgery and after Xiidra predicted the 1-month postoperative outcome with significantly greater accuracy than biometry performed before surgery and before Xiidra.9
‡‡P<0.04, paired t-test.9
“Topical lifitegrast is the first-choice immunomodulator
in the preoperative setting of 70% of ASCRS Cornea Clinical Committee members.”10
“Topical lifitegrast is the first-choice immunomodulator
in the preoperative setting of 70% of ASCRS Cornea Clinical Committee members.”10
–Starr CE, Gupta PK, Farid M, et al.
Study DesignStudy Design
A multicenter, prospective, open-label study of 100 eyes of 100 patients who were diagnosed with dry eye disease before cataract surgery. Biometry measurements were taken before surgery prior to treatment with Xiidra and again after a 28-day treatment regimen with Xiidra twice daily. The primary outcome was an improvement in the accuracy of preoperative anterior corneal power measurements at predicting postoperative spherical equivalent before and after Xiidra treatment. Secondary outcomes included changes in dry eye symptoms, as measured by SPEED scores, and dry eye signs such as corneal staining.9
Study LimitationsStudy Limitations
There was an unexplained noncompliance rate with the first course of lifitegrast of almost 20%. While reasons for discontinuation were not collected formally, anecdotal reports from patients suggested that discomfort with drop instillation was the main reason for discontinuation. Additionally, some patients did not return for their 1-month postoperative follow-up exam; however, 58 patients were enough to generate a statistical significance threshold. Some patients did not receive a second round of treatment with lifitegrast after surgery, and data were not captured on the cause for declining a second course. However, nearly the first third of study participants completed the study before the additional month of lifitegrast (postoperatively) was added to the protocol.9
Data should be interpreted with the study design and these limitations in mind. No formal conclusions should be drawn.
XIIDRA WAS WELL TOLERATED
Help patients get started by discussing AEs and the safety profile of Xiidra1
PEER PERSPECTIVES
See what experts in eye care have to say about their experience with prescribing Xiidra
References
- Xiidra. Prescribing information. Bausch & Lomb Inc.
- Holland EJ, Jackson MA, Donnenfeld E, et al. Efficacy of lifitegrast ophthalmic solution, 5.0%, in patients with moderate to severe dry eye disease: a post hoc analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2021;139(11):1200-1208. doi:10.1001/jamaophthalmol.2021.3943
- Holland EJ, Whitley WO, Sall K, et al. Lifitegrast clinical efficacy for treatment of signs and symptoms of dry eye disease across three randomized controlled trials. Curr Med Res Opin. 2016;32(10):1759-1765. doi:10.1080/03007995.2016.1210107
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a phase III, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124(1):53-60. doi:10.1016/j.ophtha.2016.09.025
- Schulze M-M, Guthrie S, Ho B, Woods J, Jones L. Do symptomatic contact lens wearers benefit from using lifitegrast? Poster presented at: TFOS conference; October 2024; Venice, Italy.
- Data on file. Bausch & Lomb Inc.
- Nichols JJ, Sinnott, LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47(4):1319-1328. doi:10.1167/iovs.05-1392
- Pucker AD, Tichenor AA. A review of contact lens dropout. Clin Optom. 2020;12:85-94. doi:10.2147/OPTO.S198637
- Hovanesian J, Epitropoulos A, Donnenfeld ED, Holladay JT. The effect of lifitegrast on refractive accuracy and symptoms in dry eye patients undergoing cataract surgery. Clin Ophthalmol. 2020;14:2709-2716. doi:10.2147/OPTH.S264520
- Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669-684. doi:10.1016/j/jcrs.2019.03.023
Indication
Xiidra® (lifitegrast ophthalmic solution) 5% is indicated for the treatment of signs and symptoms of dry eye disease (DED).
Important Safety Information
- Xiidra is contraindicated in patients with known hypersensitivity to lifitegrast or to any of the other ingredients.
- In clinical trials, the most common adverse reactions reported in 5-25% of patients were instillation site irritation, dysgeusia and reduced